
As part of our commitment to providing the most robust out-of-the-box (OOTB) offering to our safety customers, we recently…
Learn More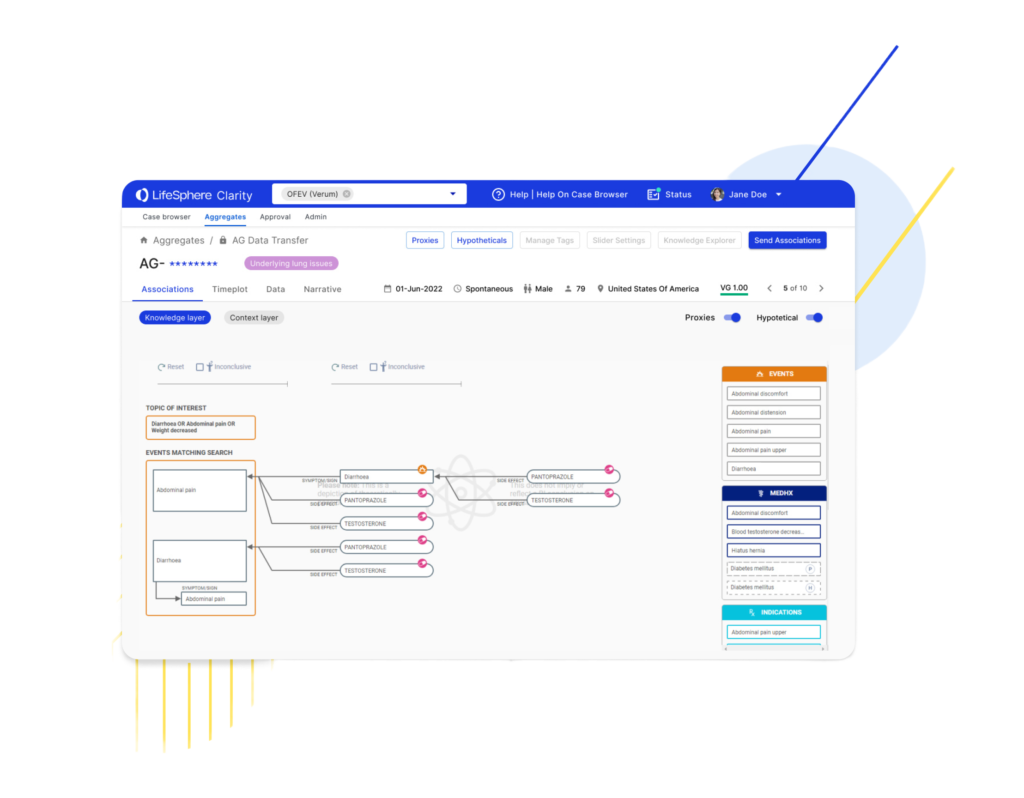

Small and emerging biopharma like yours face a crucial question – should you outsource your drug safety system, or bring…
Learn More
Regulatory Information Management (RIM) refers to an organization or team’s ability to manage…
Learn More
Every person who wants to participate in clinical research should have the opportunity to do so…
Learn More
There’s no doubt that the pharmaceutical industry is well into its ‘age of automation’. A…
Learn More
Managing your Trial Master File (TMF) is a necessary, and often tedious task for most clinical stakeholders—but it doesn’t…
Learn More
Identification of Medical Products (IDMP) has been harmonizing data globally across the drug development lifecycle since 2003. At its…
Learn More
Advances in clinical trial design and technology are significant contributors to medical progress. However, they also hamper clinical operations and…
Learn More
Managing the submission of medicinal products, medical devices, and biologics to regulatory agencies is growing more complicated all the…
Learn More