
There’s no doubt that the pharmaceutical industry is well into its ‘age of automation’. A…
Learn More
Managing your Trial Master File (TMF) is a necessary, and often tedious task for most clinical stakeholders—but it doesn’t…
Learn More
Identification of Medical Products (IDMP) has been harmonizing data globally across the drug development lifecycle since 2003. At its…
Learn More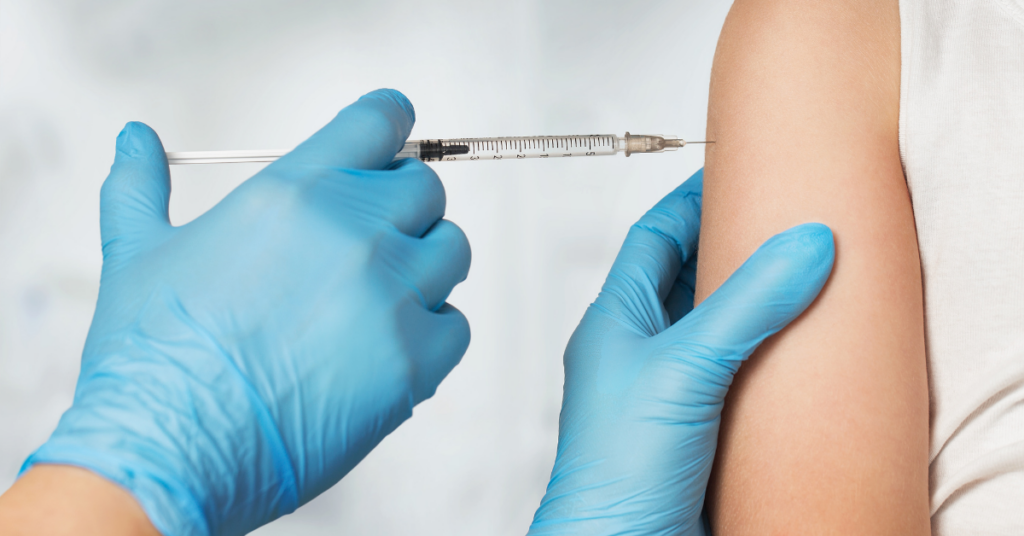
The coronavirus pandemic ushered in a new era of public scrutiny for the pharmaceutical industry. The general population was…
Learn More
Learn why Breakthrough is more than just an event but a summit for thought leaders in health and life sciences to discuss today and the future.
Learn More
Digital Impact on the Drug Safety Space Digital transformation, automation, and analytics were top of mind when ArisGlobal recently…
Learn More
The DIA Medical Affairs and Scientific Communications (MASC) Forum is created for medical affairs professionals by the best…
Learn More
Contract Research Organizations (CROs) have been enthusiastic early adopters of the LifeSphere electronic Trial Master File…
Learn More
It’s hard to think about COVID-19 and vaccine production, without reflecting on the speed, safety, and risks involved…
Learn More
As the pharmaceutical industry adapts to a new world with a new set of challenges, it is critical to keep…
Learn More